Click image to enlarge
Category: Cancer Screening (Click here to return)
Click here to request more information
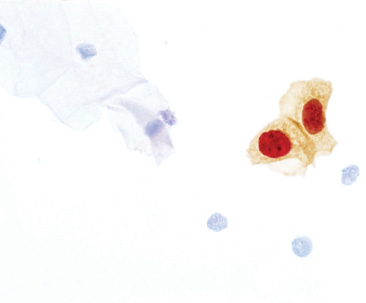
Biomarker Test for HPV and Cervical Pre-Cancer
Roche Diagnostics
Click here to view supplier website
Roche announces the US FDA approval of CINtec PLUS Cytology as a biomarker-based triage test for women whose primary cervical cancer screening results are positive for the human papillomavirus (HPV) using the cobas 4800 HPV Test. This biomarker technology simplifies clinical decision making by providing easy-to-understand results. The test identifies those patients whose HPV infections are most likely to be associated with cervical pre-cancers and enables clinicians to more confidently determine which patients should be referred to immediate further diagnostic procedures, helping to prevent patients from developing more advanced cervical disease.
Roche Diagnostics
Entry published in MLM May 2020
