- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
December 2020
Molecular Transport Media for SARS-CoV-2
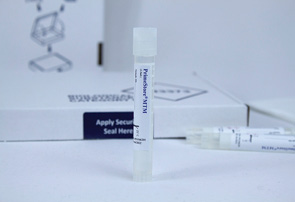
EKF Diagnostics and Longhorn Vaccines and Diagnostics announce that the novel viral transport media PrimeStore MTM (molecular transport medium) has been successfully evaluated for effective SARS-CoV-2 inactivation. After inactivation, SARS-CoV-2 material can be handled at a lower containment level, allowing more laboratories to undertake testing, and thereby increasing COVID-19 testing capacity. In addition to this successful evaluation of its effectiveness at deactivating SARS-CoV-2, the transport media is also an FDA cleared and CE-IVD marked sample collection device. PrimeStore MTM removes the need for all cold chain logistics including sample storage, as RNA and DNA are perfectly preserved for downstream molecular processing for up to 4 weeks at room temperature. Furthermore, because samples are rapidly inactivated, the risk of infection is eliminated, meaning samples are ready for safe testing immediately on arrival at a laboratory and without need for containment.
EKF Diagnostics/Longhorn Vaccines and Diagnostics, LLC