- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
September 2019
Breast Cancer Assay
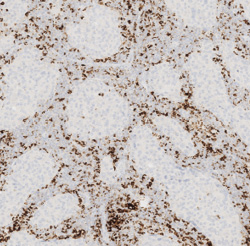
Roche has received US FDA approval of the VENTANA PD-L1 (SP142) Assay as a companion diagnostic to aid in identifying triple-negative breast cancer (TNBC) patients eligible for treatment with the Roche cancer immunotherapy Tecentriq (atezolizumab) plus chemotherapy (Abraxane [paclitaxel protein-bound particles for injectable suspension (albumin-bound); nab-paclitaxel]). Assessment of PD-L1 biomarker status on tumor-infiltrating immune cells with the assay is essential for identifying those patients most likely to benefit from the treatment. A diagnosis of triple-negative breast cancer means that the three most common proteins associated with breast cancer growth (estrogen receptor, progesterone receptor, and HER2/neu) are not expressed on the tumor. The assay was developed to enhance visual contrast of tumor-infiltrating immune cell staining. In cases of TNBC, PD-L1 is primarily expressed on tumor-infiltrating immune cells rather than on tumor cells themselves.
Roche Diagnostics