- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
December 2020
Blood Cell Differentials for Low-Volume Labs
Sysmex Corporation
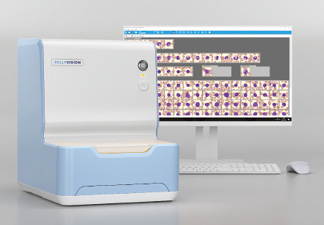
Sysmex America announces US FDA 510(k) clearance for the CellaVision DC-1, which automates and simplifies the process of performing blood cell differentials in low-volume hematology labs. Eliminating most manual steps traditionally done by lab technicians performing blood cell differentials maximizes speed and productivity, and the system’s standardized testing process helps reduce the negative impact of subjective interpretations and assessments. In addition, the device reduces review time for differentials, standardizes differential results by automating cell location and pre-classification, and enables input from across the organization through real-time collaboration and centrally stored images and comments.
Sysmex America
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 MLM Magazine - MedicalLab Management.
All rights reserved.