- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
January 2021
SARS-CoV-2 Antibody Tests
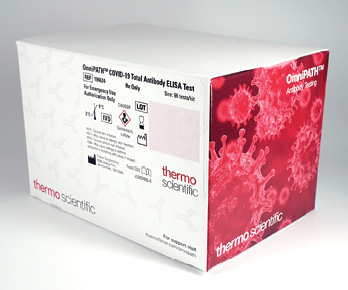
Thermo Fisher Scientific announces the launch of a new portfolio of SARS-CoV-2 antibody tests, including the OmniPATH COVID-19 Total Antibody ELISA test and the EliA SARS-CoV-2-Sp1 IgG test. The OmniPATH ELISA test has been granted an EUA from FDA, authorizing qualitative detection of total antibodies to SARS-CoV-2 in human serum when run manually or using the Dynex AGILITY automated ELISA workstation. Developed in conjunction with Mayo Clinic and WuXi Diagnostics, it detects three major antibodies (IgM, IgA, and IgG) to help clinicians determine exposure to SARS-CoV-2. The EliA SARS-CoV-2-Sp1 IgG test is semi-quantitative in the US and is available on the Thermo Scientific Phadia 250 instrument, designed for automated processing of up to 60 results per hour. This antibody test portfolio is designed to meet the need for open ELISA and automated workflows. The tests’ flexibility enables laboratories to run the tests at customizable speed and throughput while using automated instruments already in place, minimizing initial costs and reducing the time needed to begin testing.
Thermo Fisher Scientific