- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
October 2018
Glycemic Control Assay Kit
EKF Diagnostics
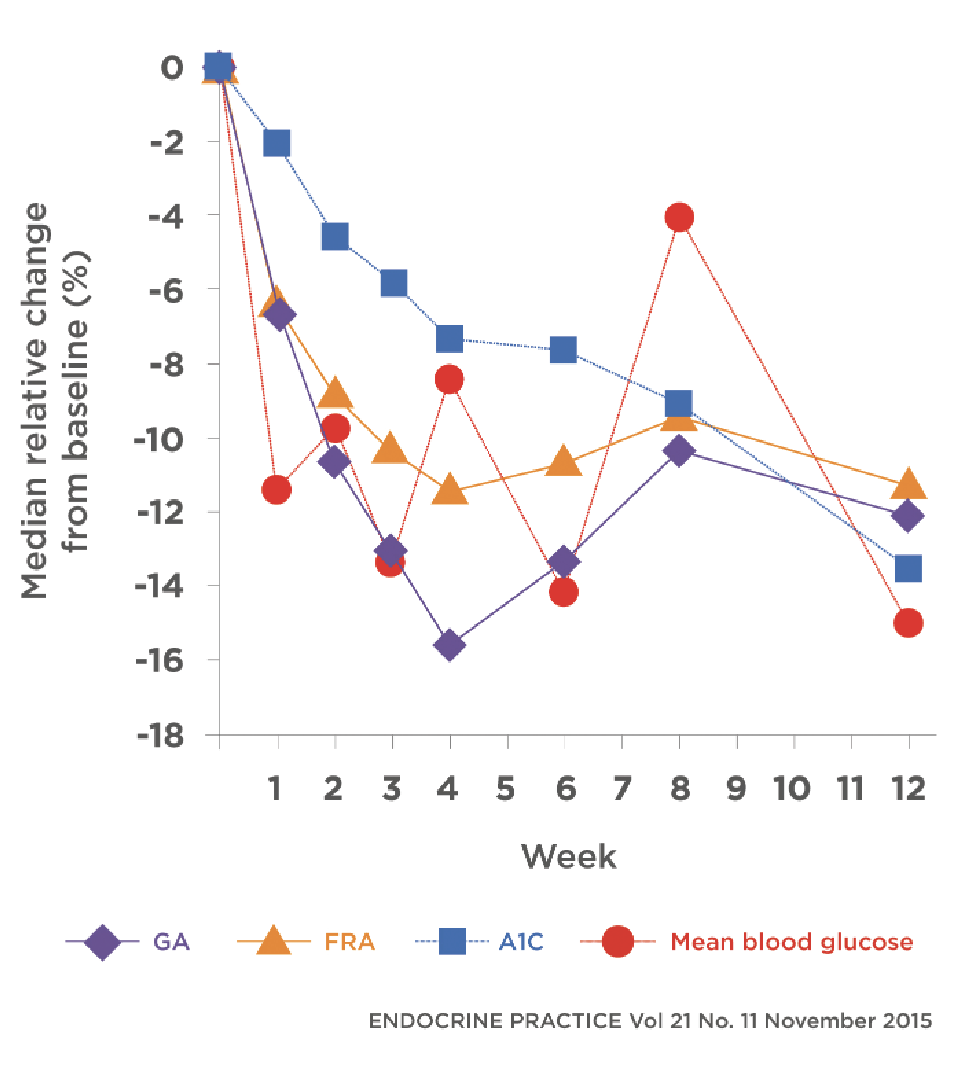
EKF Diagnostics introduces the Stanbio Chemistry Glycated Albumin (GA) assay kit, now with FDA 510(k) clearance for sale in the United States. GA is a sensitive marker for intermediate glycemic control in diabetes and can fill the gap between long- and short-term measurements of blood glucose status. The assay kit uses an enzymatic method with liquid reagents and requires no preparation. It can be used on compatible open channel clinical chemistry analyzers and expresses results as a ratio of glycated albumin to albumin in mmol/mol from a small amount of blood serum. GA accurately reflects the latest blood glucose status as it is found throughout the body and is a type of protein that can be readily glycated.
EKF Diagnostics
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 MLM Magazine - MedicalLab Management.
All rights reserved.