- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
March 2020
CPO Detection Assay
BD
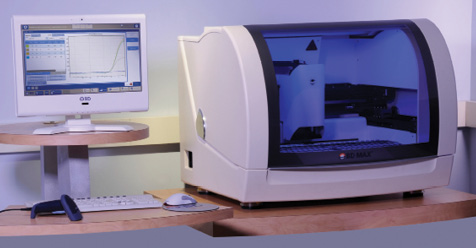
BD announces FDA approval for its Check-Points CPO assay for the BD MAX fully automated molecular platform. Created to help manage antibiotic-resistant hospital-acquired infections (HAIs), BD’s molecular panel can detect 5 of the most common carbapenemase genes in 2.5 hours. This detection assay is an addition to BD’s portfolio dedicated to reducing transmission and outbreak of deadly pathogens, allowing hospitals and clinical laboratories to offer efficient time to result, and enabling immediate deployment of control measures and treatment.
BD
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 MLM Magazine - MedicalLab Management.
All rights reserved.