- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
July 2024
Digital Pathology Platform
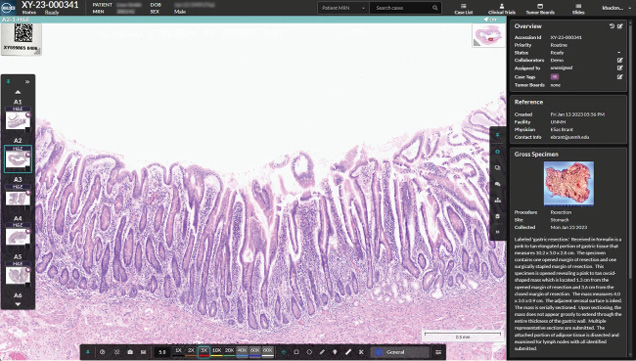
Indica Labs has received FDA clearance for HALO AP Dx, its digital pathology platform for primary diagnosis. This 510(k) clearance allows HALO AP Dx to be utilized in conjunction with the Hamamatsu NanoZoomer S360MD slide scanner for in-vitro diagnostic use. As an interoperable platform, the HALO AP Dx can be incorporated into the lab’s existing workflows, helping to create a custom-built environment for digital pathology and for a variety of pathologic specialties, even those with unique technical requirements. The system can be deployed in either a cloud-based or on-premises environment. In addition to streamlining the diagnostic process, HALO AP Dx enables remote work and remote consultations—features that assist facilities affected by staffing shortages. With slides available for review from any location the moment they are scanned, pathology labs can run an uninterrupted service with around-the-clock, global collaboration without having to ship a single slide.