- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
July-August 2018
Validation and Competency Kit
Hemo bioscience
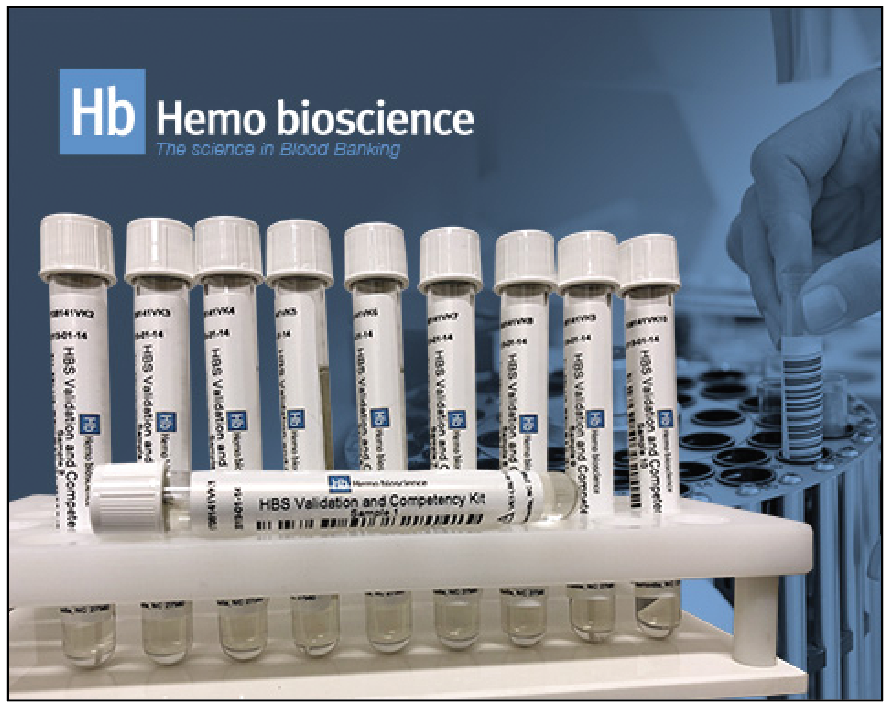
Hemo bioscience introduces the Validation and Competency Kit. The kit comprises a panel of samples that serves as material to validate the blood typing, antibody detection and identification, and compatibility testing capabilities of instrument test systems and testing technologists. It is available for use on all platforms and is suitable for tube, gel, solid phase, and manual or automated methods. It includes ten samples, features a 2-year shelf life, and offers a large fill volume. In addition, the kit includes instructions so that laboratories may validate the cross matching compatible and incompatible features of their instruments.
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 MLM Magazine - MedicalLab Management.
All rights reserved.