- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
January 2021
Strep A Test
Mesa Biotech
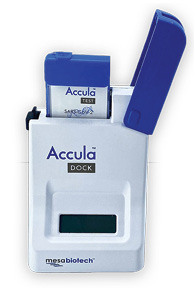
Mesa Biotech has received 510(k) clearance and CLIA waiver from FDA for its Accula Strep A test. The Strep A cassette, for the molecular detection of Group A Streptococcus bacterial nucleic acid by rapid PCR, is cleared for diagnosing both children and adults, and provides laboratory quality results in 30 minutes at the point of care. As the third molecular POC diagnostic on the Accula platform to receive FDA clearance and CLIA waiver, the ergonomically designed, simple to use test fits in the palm of the hand. It is visually read, using reverse transcription-polymerase chain reaction (RT-PCR) technology to detect Strep A via throat, nasal, or nasopharyngeal swab samples.
Mesa Biotech
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2024 MLM Magazine - MedicalLab Management.
All rights reserved.