- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
May 2019
Sepsis Indicator
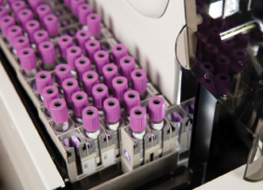
Beckman Coulter’s Early Sepsis Indicator, a hematology-based cellular biomarker designed to help identify patients with sepsis (or those with an increased risk of developing sepsis) has received US FDA 510(k) clearance. In clinical trials, the system discriminated sepsis from other conditions when combined with the current standard of care. A positive result signals a higher probability of sepsis, enabling physicians to initiate lifesaving treatments quickly; conversely, a negative reading indicates a lower probability of sepsis. The system can be used with Beckman Coulter’s Multidiscipline Reflex Rules in REMISOL Advance middleware. These reflex rules allow for the creation of customized, automated reflex panels featuring Beckman Coulter’s portfolio of in vitro diagnostic tests in the current sepsis identification and management care pathway across multiple disciplines.
Beckman Coulter Diagnostics