- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
September 2017
Quality Control for CAT Testing
Hemo bioscience
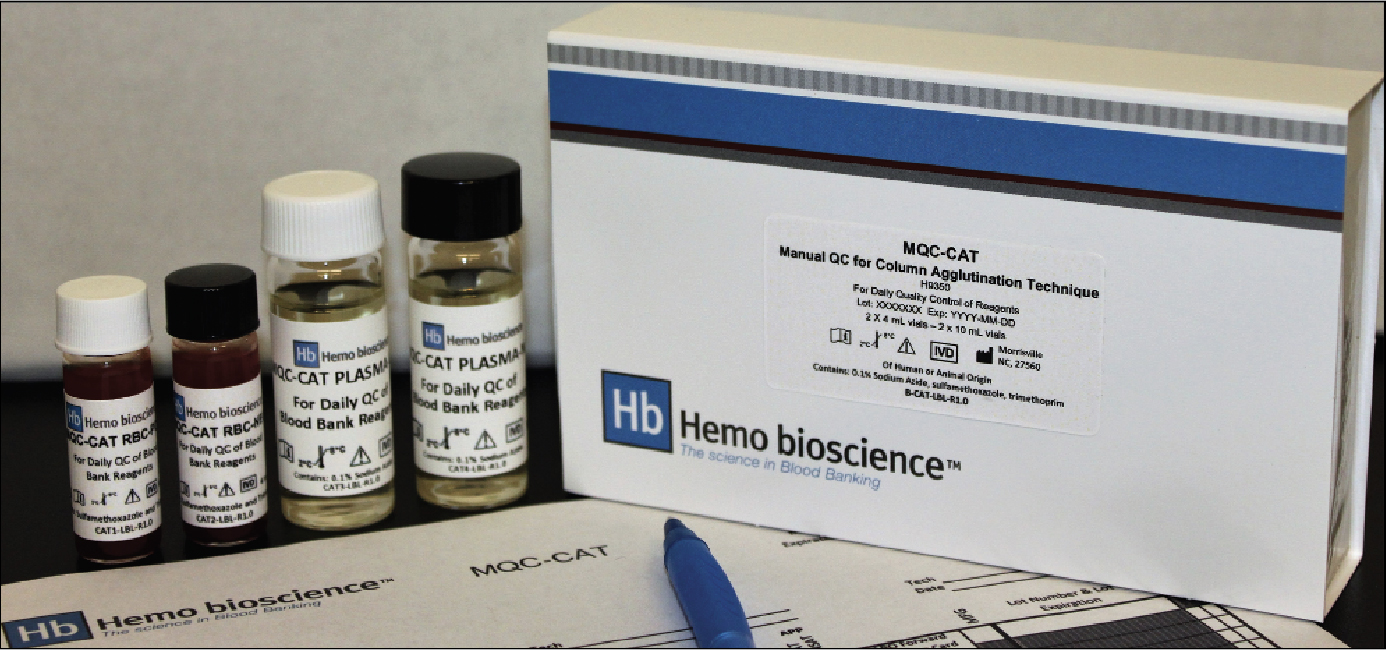
Hemo bioscience is pleased to announce that MQC-CAT, a ready-to-use quality control product for manual column agglutination technology (CAT) blood bank testing methods, has received US FDA 510(k) clearance. Validated for use in all manual CAT testing systems currently available in the US, MQC-CAT includes both positive and negative red cells and serums to ensure that ABO and Rh(D) as well as antibody screening and identification reagents are performing as expected.
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2024 MLM Magazine - MedicalLab Management.
All rights reserved.